By Nathan Boyd
A fellowship from the California Institute for Quantitative Biosciences (QB3) launched Damon Wheeler, a graduate student in Jin Zhang’s physical chemistry lab at UC Santa Cruz, in research on nanoparticles and their potential role in cancer therapy.
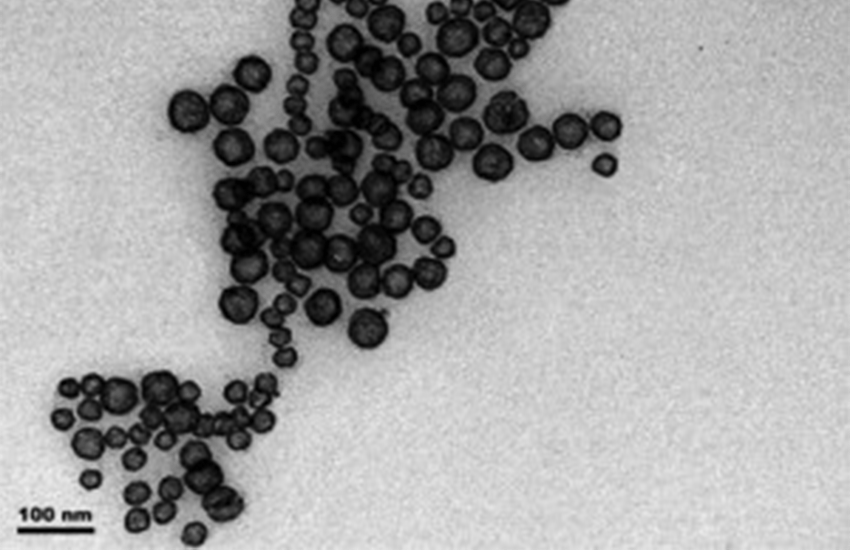
Wheeler characterizes the optical properties of hollow gold nanospheres (HGNs) to discover how they can be effectively used to obliterate cancer cells and toxic molecules upon irradiation with precisely focused light.
In 2005, a group of researchers in Beijing created the first HGNs without fully describing their properties.
The Zhang group was drawn to the new particles as an undescribed species with untapped potential, so they set out to characterize the optical behavior of HGNs and explore applications for them.
With a background in chemical engineering from New Mexico Tech, Wheeler joined the Zhang group last year with a one-year fellowship from QB3 to study HGNs. The fellowship gave Wheeler access to the QB3 experts, instrumentation, and facilities at UC Santa Cruz, UC San Francisco, and UC Berkeley.
“The fellowship is a vehicle for cross-talk between QB3 departments,” Wheeler said. “That interaction was truly fundamental to our work.”
The Zhang lab showed that HGNs can be manufactured in the lab for downstream experiments by reacting cobalt with gold salt in aqueous solution. Employing a galvanic replacement reaction, gold ions are reduced to elemental gold on pre-formed cobalt nanoparticles. Simultaneously, the elemental cobalt is oxidized and leaves the complex.
The amount of gold added during synthesis modulates the HGN’s aspect ratio–the relationship of sphere diameter to shell thickness. HGNs with different aspect ratios react differently to radiation, the property of interest for potential applications in living systems.
Wheeler has discovered that an aspect ratio of 10:1 (sphere diameter of 50 nm to shell thickness of 5 nm) yields absorbance spectra in the visible and near infrared range, a comfortable range for most biological processes.
When HGNs are hit by focused laser light, the electrons in the gold atoms oscillate collectively–a condition known as surface plasmon resonance.
The surface plasmon resonance created by a specific frequency of light tailored to the HGN’s aspect ratio is enough to create a radius of destruction around the HGN, including the sphere’s architecture and surrounding matrix.
Once they have manufactured a useable batch of HGNs, Zhang’s team sets about to destroy it with lasers, a strategy that could be useful for obliterating a variety of undesirable biological entities that surround or are connected to the HGN.
In a recent publication in the Journal of Physical Chemistry, Zhang’s group showed that it is indeed possible to selectively destroy HGNs based on absorbance of electromagnetic radiation.
If HGNs could be attached to the surface of a cell or absorbed into it, the intense atomic rumbling of an excited HGN could be enough to destroy it along with adjacent cells or cell systems.
Wheeler said, “Human tissue is relatively transparent to near infrared radiation, so the idea is that HGNs locally heat, vibrate, and destroy the encapsulating matrix, while leaving other biology undisturbed.”
Indeed, collaborators at the University of Texas MD Anderson Cancer Center have shown promising evidence that the Zhang model for photothermal ablation of HGNs can be deployed to destroy melanoma.
In addition to their possible use in photothermal ablation, Wheeler said, HGNs could also be used as a Trojan horse drug-delivery system in which nanospheres encapsulate cytotoxic cocktails for targeted delivery to specific cells in the body, spilling their contents when irradiated.
Few labs study the optical properties of HGNs, and significant challenges remain, including expansion of the HGN detection limit and increasing the efficiency of energy transfer between the lasers and the HGNs.
Wheeler explains that nanotechnology offers unique characteristics that make it useful in tackling large biological problems. “HGNs may offer a more selective, ‘fine scalpel’ approach to cancer treatment than traditional cytotoxic methods.”